






Dr. Shinde is the full-time laboratory director supervising all laboratory functions. Dr. Shinde is board certified in both Anatomic and Clinical Pathology by the American Board of Pathologists.
She brings more than 16 years of laboratory management and clinical experience to our PCR laboratory.

3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri-Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
1601 W Division
Chicago, IL 60622
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri-Sat: 8am – 5pm CST
Sun: 10am – 4pm CST
3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri-Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
647 W Randolph St
Chicago, IL 60661
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 5pm CST
Fri-Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
1601 W Division
Chicago, IL 60622
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri-Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri – Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
1601 W Division ST
Chicago, IL 60622-3809
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri – Sat: 8am – 5pm CST
Sun: 10am – 4pm CST
3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri – Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
647 W Randolph St
Chicago, IL 60661
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 5pm CST
Fri – Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
1601 W Division ST
Chicago, IL 60622-3809
(424) 282-6843
Hours of Operation
Mon – Thur: 8am – 7pm CST
Fri – Sat: 8am – 5pm CST
Sun: 9am – 5pm CST
Results are available in 24-48 hours.
Simply schedule online and then come to any of our locations at Lakeview, Wicker Park or West Loop (add link to the location for a pop-up with map)
We are performing nasal swabs for COVID PCR test as well as antibody test in Chicago, IL. We are testing people with and without symptoms. We expect the rapid COVID test in 10 days but are not offering the rapid test at present.
We are coronavirus testing all those interested in knowing their results
if you may have COVID-19 infection
if you are an asymptomatic carrier
if you would like to know whether you have antibodies to COVID-19
To get tested, please schedule a brief telemedicine visit to determine the best test for you at which time, we will schedule your testing.
Please schedule your visit and we will determine the best test for you upon arrival!
Symptoms of COVID-19 appear anywhere from 2 to 14 days after exposure.
Symptoms that may appear 2-14 days after exposure to the virus:
Cough
Shortness of breath or difficulty breathing
Or at least two of the following:
Fever
Chills
Repeated shaking with chills
Muscle pain Headache
Sore throat
New loss of taste or smell
If you develop any of these emergency warning signs for COVID-19, get emergency medical attention immediately: Trouble breathing
Persistent pain or pressure in the chest
New confusion or inability to arouse
Bluish lips or face
Average incubation 5-6 days (range 1-14)
From onset to max illness – usually about a week.
Recovery is about 2 weeks for mild to moderate disease but 3-6 weeks for severe disease.
Active COVID-19 infection Day 1-14 after symptom onset -nasal swab RT-PCR testing – allows for early case identification and informing close contacts, reducing spread at home or work
IgM antibody response to COVID-19 Day 7-21
IgG antibody response to COVID-19- after day 14- informs of the presence of an antibody response
Importantly, individuals who had a mild flu like illness that was in fact, COVID-19 -these individuals would have antibodies.
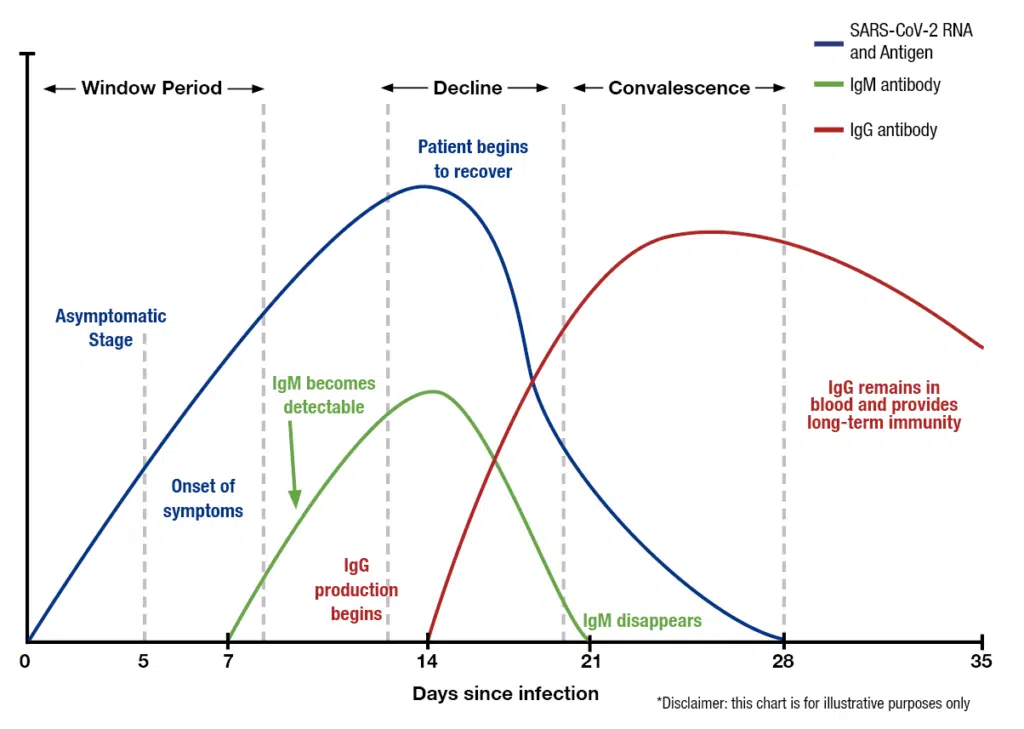
Timing Is Critical For Correct Testing
Day 1-14 since onset of symptoms: Nasal swabs – COVID-19 (RT-qPCR)
Day 14-21 after symptom onset: Nasal swab + IgM antibody blood test for COVID-19
After Day 21 after symptom onset: IgG antibody blood test for COVID-19
Antibody testing should be performed only in individuals after symptom resolution to avoid false-negative results
There are now two ways to test for Covid-19: one that measures the actual virus, and one that measures the body’s reaction to the virus.
The first is a genome test that detects the presence of viral genes in the body using a swabbed sample, usually taken from the nose and throat. The sample is then sent to a lab where it can be replicated and analyzed in a PCR machine. RT-PCR technology plays a role especially in the early stage of a viral infection, in which the virus reproduces quickly. Results take anywhere from one to three days to deliver. Point-of-care genome tests have also been developed that can potentially shorten this waiting period to mere minutes however, there accuracy at this time is questionable.
The second, which only recently has received FDA approval in the United States, is an antibody test, or serology test, that detects the presence of SARS-CoV-2 specific antibodies in the blood. Instead of a nasopharyngeal swab, results are obtained using a finger prick blood test. Like the nasal swab test for COVID-19 viral genes, the sample is processed in an FDA approved high-throughput lab and results come back in one to three days.
A triple epidemic has been warned about due to COVID, the early increase in flu cases this season, and RSV. However, how the three illnesses will interplay as the fall season develops is still being determined.
During flu season, it’s possible for the viruses that cause COVID-19 and the flu, or perhaps even RSV, to spread in one’s surroundings simultaneously. Individuals may be coinfected with one or both diseases if this occurs.
It is challenging to distinguish between the COVID-19, RSV, or the flu since they have similar symptoms; co-testing is the best approach to determine which infection you have.
Rapid antigen and RT-PCR tests differ in their analytic sensitivity. Typically, the sensitivity of antigen tests is 30% to 40% lower than for RT-PCR, depending on whether tested subjects were symptomatic or asymptomatic [reference]
However, lower sensitivity has both disadvantages and benefits.
The primary disadvantage is a risk of falsely negative results in people with low viral loads who may be early in their infection, and who go on to spread it to others in subsequent days. In practice, this subpopulation represents just a small fraction of those tested, and risk can be mitigated through serial testing algorithms. There is also a slightly elevated rate of false positives relative to molecular tests, though the rate is dependent on the prevalence of disease and the proportion of people who are symptomatic. A paper published in Lancet, Infectious Diseases noted that for many commonly-used rapid antigen tests, the negative predictive value (e.g., the likelihood someone with a negative test is truly negative for infection) is greater than 98%. [reference]
A major advantage of these assays is the lower likelihood of detecting residual viral nucleic acid left over from a remote infection in recovered individual. This reduces the chance of unnecessary initiation of isolation and quarantine precautions and subsequent rounds of testing.
Rapid antigen tests are subject to the same considerations as the PCR tests with respect to factors that affect clinical sensitivity. These include the quality of sampling and the timing of testing relative to the onset of infection. The CDC advises that rapid antigen tests can have a lower performance in the asymptomatic population, which may be related to the lower levels of virus in this group relative to those with symptoms, rather than characteristics of the tests themselves [reference]
Nucleic Acid Amplification Tests (NAATs) such as RT-PCR for SARS-CoV-2 are designed to detect viral RNA. A positive result is highly specific for the presence of COVID-19 viral nucleic acid. However, it is very important here to note that a positive RT-PCR test does not differentiate between viable and nonviable virus. Thus, a positive test does not necessarily indicate that a person is infectious and requires isolation.
A variety of gene targets are available across different assays, including the envelope gene, the nucleocapsid gene, the ORF1ab gene, and the spike gene. Most assays are designed to detect two or more gene targets. The FDA maintains an up-to-date list of assays with emergency use authorization. [reference]
The RT-PCR’s clinical sensitivity is affected by the assay’s limit of detection, the time since infection began, and sample type. Because patients with symptoms tend to present earlier in their infection course, there is increased test sensitivity in symptomatic people relative to asymptomatic people. Traditionally the nasopharynx has been the preferred body site for sampling, but given patient discomfort and the need for serial testing, many hospitals, and public health programs now use anterior nares or saliva as the primary sampling type.
All symptomatic individuals suspected of having COVID-19;
Asymptomatic individuals with known or suspected contact with a COVID-19 case;
Asymptomatic individuals when the results will impact isolation/quarantine/personal protective equipment usage decisions, dictate eligibility for surgery, or inform the administration of immunosuppressive therapy.
NAAT testing in symptomatic people, even when clinical suspicion is low;
NAAT testing in asymptomatic individuals with known or suspected exposure to COVID-19, those requiring hospitalization when community prevalence is high or prior to solid organ or stem cell transplantation and time-sensitive surgeries;
Repeating viral RNA testing when the initial test is negative in symptomatic individuals with intermediate or high clinical suspicion;
Specimen collection from the nasopharynx, mid-turbinate, anterior nares, or combined nasal/oropharyngeal over oropharynx alone.
The COVID-19 antibody test looks for IgM and IgG antibodies in the blood to determine if your body has already responded to the novel coronavirus or if you have not developed antibodies (you have a higher risk of getting the virus if you do not have antibodies).
Nasal swab: Quest Diagnostics performs the nasal swab RT-PCR. Quest reports that the concentration of SARS-CoV-2 RNA that was successfully detected with at least a 95% detection rate was calculated as 136 copies/mL. It is important to note that timing and adequate sampling determine this test’s sensitivity. Recent data show that RNA positive rates peaked in upper respiratory tract specimens at 7–10 days after symptom onset and then steadily declined after that. Sensitivity also depends on an adequate sample being collected and the disease having advanced enough to have a higher number of viral RNA copies.
IgG antibodies: We offer the Roche Elecsys Anti-SARS-CoV-2 igG Total Antibody test. Roche reports an overall test specificity of 99.81 % (99.65 – 99.91 %) and a sensitivity in patients 14 days after PCR confirmation to be 100% (88.1 – 100 %).
Both, Quest Diagnostics and Roche offer testing in accordance with the FDA Emergency Use Authorization Guidance.
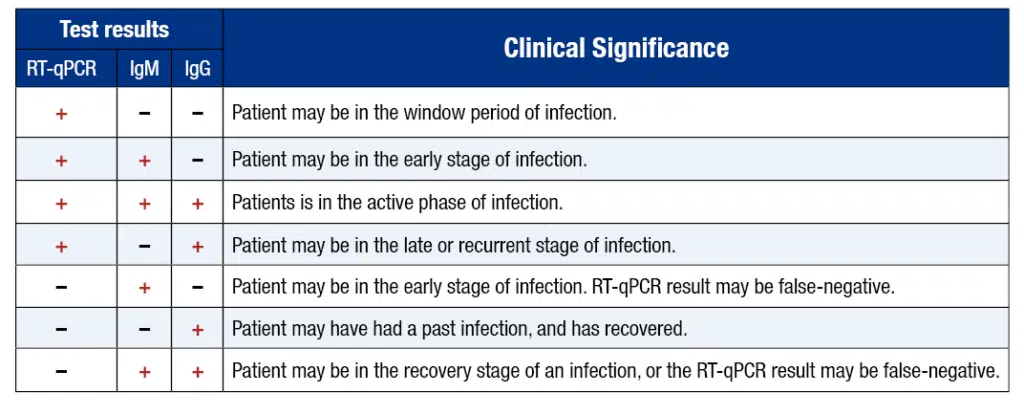
Antibody tests, also known as serology tests, differ from the nasal- and throat-swab tests that can spot a current infection. Instead, these look for signs in the blood that the immune system had fought off an infection in the past.
But there are limits to what antibodies can reveal. With other infectious diseases, antibodies signal a measure of protection against getting sick again. No one knows if that is true of this virus, or, if it is, how long such immunity might last.
A positive test result with the qSARS-CoV-2 IgG/IgM test indicates that antibodies to SARS-CoV-2 were detected, and the patient has potentially been exposed to COVID-19. When IgM antibodies are present, they can indicate that a patient has an active or recent infection with SARS-CoV-2. IgG antibodies develop later following infection, and generally do not begin to appear until 14-21 days after infection. When IgG antibodies are present it, often indicates a past infection but does not exclude recently infected patients who are still contagious, especially if detected with IgM antibodies.
Since most patients do not know the exact time when they contracted the virus, the combining both IgM and IgG tests provides a much higher test sensitivity and test specificity.
The presence of antibodies and immunity
It is unknown how long IgM or IgG antibodies to SARS-CoV-2 will remain present in the body after infection and if they confer immunity to infection.
If you think you might have been exposed to the COVID-19, please schedule your appointment immediately.
COVID-19, Flu & RSV Nasal Swab RT-PCR Test: The sensitivity of this test is low in early illness, and is even lower in asymptomatic individuals, likely because of lower viral load, which means even more false negatives. The test’s sensitivity is also dependent upon obtaining an adequate sample from inside the nose. Sensitivity of RT-PCR is likely to be higher later in the illness when the viral load is higher.
COVID-19 & Flu Antibody Tests: Antibodies may not be present in detectable levels in early days of an infection. Test sensitivity increases with the time from from onset of symptoms and is more sensitive later. Delaying a test for too long may not be fruitful because at this time, we do not know how long IgM or IgG antibodies to SARS-CoV-2 or Influenza A & B will remain present in the body after the infection has been cleared.
TESTING IS FREE IF YOU ARE A US RESIDENT AND WE ACCEPT YOUR INSURANCE:
A). You have health insurance AND and its accepted by us: Your insurance will cover all costs of evaluation & COVID testing. We accept a wide variety of insurance plans for covid testing.
B). You do not have health insurance – The CARES act will NOT COVER the evaluation & coronavirus testing costs for anyone without insurance as of March 21, 2022.
IF A OR B DO NOT APPLY TO YOU:
AND YOUR INSURANCE COVERAGE IS BY MEDICAID, PLEASE CLICK HERE FOR PRICING.
AND YOU DO NOT HAVE INSURANCE AND DO NOT HAVE A U.S. SSN# PLEASE CLICK HERE FOR PRICING.
Lakeview
3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Wicker Park
1601 W Division
Chicago, IL 60622
(424) 282-6843
Open 7 Days
Mon-Thu: 8a-7p
Fri-Sat: 8a-5p
Sun: 9a-5p
Lakeview
3264 N Lincoln Ave
Chicago, IL 60657
(424) 282-6843
Wicker Park
1601 W Division
Chicago, IL 60622
(424) 282-6843
Open 7 Days
Mon-Thu: 8a-7p
Fri-Sat: 8a-5p
Sun: 9a-5p
This website uses cookies to ensure you get the best experience. See our Privacy Policy to learn more.